 With Nonalcoholic Fatty Liver Disease on the Rise and No NHS-Approved Treatment, Nutrient and Botanical Therapies Are Poised as the Next-Best Solutions
With Nonalcoholic Fatty Liver Disease on the Rise and No NHS-Approved Treatment, Nutrient and Botanical Therapies Are Poised as the Next-Best Solutions
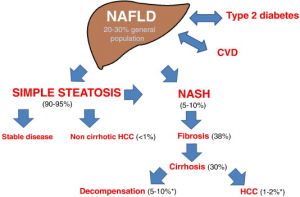
As we move well into the 21st century, diseases related to a sedentary lifestyle and an overabundance of food are increasingly common. The rate of type 2 diabetes (T2D), a condition that was estimated to affect 120 million people worldwide in 2000,[1] has quadrupled in global incidence over the last three decades.[2] Diabetes and obesity are often accompanied by nonalcoholic fatty liver disease (NAFLD), a condition characterised by fat, in the form of triglycerides (TGs), accumulating in the liver.[3] Nonalcoholic steatohepatitis (NASH), a more severe form of NAFLD that also involves inflammation, was first described medically in 1952 and only identified as a disease in 1980.[4],[5] Nowadays, recent surveys have shown that about 30 to 40% of adults have NAFLD and 3 to 12% are affected by NASH.[6],[7] The numbers are shockingly high in those who are obese or affected by diabetes: 30 to 90% of individuals who are obese and 60 to 75% of individuals with T2D have NAFLD.[8],[9],[10]
Children are also increasingly affected by these conditions. The incidence of T2D in children has been shown to be increasing by 4.8% yearly, compared to a yearly increase of only 1.8% of type 1 diabetes.[11] At this rate, by the close of the century, T2D will likely overtake autoimmune diabetes as the most common form of paediatric diabetes. In some regions and age groups, T2D already accounts for more than half of all cases of paediatric diabetes.[12] NAFLD, first reported in the paediatric population in 1983,.[15] Genetic susceptibility causes this disease to have a heritable aspect[16],[17] and gives reason for further scrutiny and screening in children whose parents are affected, particularly when they do not present with the typical coexisting conditions of obesity and diabetes.
The role that gut health and dietary choline deficiencies may play in the development of NAFLD are discussed at length in the Summer 2018 issue of FOCUS.[18] The evidence behind directed interventions like supplemental choline,[19] probiotics,[20] and berberine[21] (an alkaloid derived from botanicals such as Oregon grape and barberry) is also reviewed therein. In this issue, we discuss evidence behind vitamin E (both as alpha tocopherols and a blend of delta and gamma tocotrienols), milk thistle seed extract, and essential fatty acids as tools in the fight against NAFLD. We also discuss additional research pertaining to treatment of pediatric NAFLD with probiotics and choline.
Vitamin E
As oxidative stress and diminished antioxidant defences are factors leading the development of NAFLD,[22] it should not be surprising that the use of antioxidants has been investigated as a potential treatment for the condition.
Vitamin E has been studied in several clinical trials for the treatment of NAFLD, both as a standalone and as an adjunctive therapy. In a review of these studies, the dosage of vitamin E was 400 to 1,200 IU/day with study durations from 24 weeks to more than two years.[23] Findings generally were positive, showing that vitamin E supplementation was associated with improvements in histology, steatosis, and/or transaminase levels.[24],[25] In an open-label pilot study including 11 children with NAFLD, supplementation of between 400 to 1,200 IU of vitamin E for four to 10 months was found to normalise transaminase and alkaline phosphatase levels during treatment, yet these parameters returned to abnormal ranges once treatment was stopped.[26] Hepatic echogenicity did not change during the course of treatment.
Tocotrienols are the lesser-studied family of the naturally occurring forms of vitamin E, with tocopherols, more often found in nature, dominating scientific research.[27] Numerous clinical findings indicate that, like tocopherols, tocotrienols may be beneficial for reducing fatty liver changes. Tocotrienols have been shown to improve total cholesterol and its fractions, reducing total and low-density lipoprotein (LDL) cholesterol by 15 to 20%[28],[29] and TGs by up to almost 30%.[30] Tocotrienols have also been shown to have anti-inflammatory and antioxidant effects in vivo, lowering high-sensitivity C-reactive protein (hs-CRP) levels and reducing LDL oxidation.
In adults with ultrasound-diagnosed NAFLD, 200 mg of mixed tocotrienols (sourced from sustainable palm oil, with a high gamma fraction and additionally providing 61 mg of alpha-tocopherol) taken twice daily for one year was shown to significantly normalise hepatic echogenic response and rate of remission compared to placebo.[31] In a second study of patients with ultrasound-diagnosed NAFLD and transaminase elevation, 300 mg of tocotrienols (a 90:10 delta:gamma blend) taken twice daily for 12 weeks significantly decreased AST, ALT, hs-CRP, and malondialdehyde (a marker of oxidative stress) levels, as well as fatty liver index score, compared to placebo.[32]
Milk Thistle
Milk thistle (Silybum marianum) is well known for its liver-protective effects. Not surprisingly, this botanical has also been investigated for the treatment of NAFLD in several clinical studies. The active compounds found in milk thistle, silybin and silymarin, have been shown to activate a nuclear bile acid receptor known as farnesoid X receptor (FXR) in hepatocytes. FXR regulates bile acid, glucose, and lipid metabolism[33]—each of which plays a role in liver health. Activation of FXR by silymarin has been shown to down-regulate inflammatory pathways and metabolic dysfunction induced by high-fat diet (HFD) feeding.[34] Medications that interact with FXR in a similar manner to these milk thistle–derived compounds are also being investigated for the treatment of NAFLD.[35] Silymarin has additionally been shown to increase both hepatic and intestinal glutathione levels, which tend to be lower in individuals with NAFLD.[36],[37]
Clinical studies have shown milk thistle improves various parameters associated with NAFLD. A 2017 meta-analysis found that treatment with milk thistle significantly reduces ALT and AST by 5.08 IU/L and 5.44 IU/L, respectively, in patients with NAFLD.[38] Dosages ranged from 140 mg once a day to 200 mg three times a day, for a duration of eight to 24 weeks. After eight weeks at the lowest dosage of 140 mg daily, significant improvements were seen in fasting blood glucose (FBG), lipid profiles, and serum insulin levels; additionally, AST and ALT were reduced from 56 to 37.77 IU/L and 78.73 to 53.05 IU/L, respectively.[39]
Milk thistle has also been investigated for the treatment of NAFLD in children ranging from five to 16 years of age.[40] In this population, silymarin was provided in divided dosages at mealtime with a total dose of 5 mg/kg/day. Children diagnosed with NAFLD (based on history, physical examination, liver sonography, and liver enzymes) were randomised into two groups, with the control and intervention groups both being recommended lifestyle interventions (LI) of 150 to 250 minutes of walking a week and a low-fat and low-carbohydrate diet. After 12 weeks of the interventions, the children receiving silymarin in addition to LI had a significantly lower grade of fatty liver and significantly improved AST and ALT levels, while none of these parameters changed significantly in the control group.
A combination of milk thistle and vitamin E has also been assessed in clinical studies for the treatment of adults with NAFLD.[41],[42] Again, participants in both the control and intervention groups were assigned LI (hypocaloric diet and regular exercise) while the intervention group also received the combination of milk thistle and vitamin E. In the larger of these two studies,42 a regimen of 420 mg of silymarin (approximately 60% silybin) and 60 IU of vitamin E (form unspecified) taken daily for 90 days was associated with significantly greater reductions in the abdominal circumference, body mass index (BMI), and ultrasound-measured size of right liver lobe, as well as the hepatic steatosis and lipid accumulation indices.
Essential Fatty Acids
Due to their effect on TG levels and inflammation,[43] essential fatty acids (EFAs) have also been the topic of numerous clinical studies for the treatment of both adult and paediatric NAFLD. Supporting this research further, dietary assessment of children with NAFLD has shown a lower consumption of fish (which provides EFAs) and supplemental EFAs than controls.[44]
Multiple meta-analyses investigating the impact of EFAs on NAFLD have been performed,[45],[46],[47] including one that solely looked at paediatric research.[48] The primary findings of these larger analyses, which included daily dosages ranging from 250 mg of docosahexaenoic acid (DHA) to a 50 mL mixture of eicosapentaenoic acid (EPA) + DHA, all supported the use of EFAs for the treatment of NAFLD. A pooled estimate from 11 randomised, controlled trials (RCTs) concludes that essential fatty acids significantly reduce ALT (by 7.53 IU/L), AST (by 7.10 IU/L), and TGs (by 36.16 mg/dL), and marginally reduce liver fat by (5.11%).47 In this same evaluation of the 11 RCTs, a dose-response analysis showed incremental decreases of ALT (3.14 IU/L/g EPA + DHA), AST (2.43 IU/L/g EPA + DHA), liver fat (2.74%/g EPA + DHA), and TGs (9.97 mg/dL/g EPA + DHA) with EFA supplementation. An improvement of histology was also seen in many of the studies that assessed this parameter.45 In children, supplementation of EFAs (with dosages ranging from 250 to 1,300 mg/day) was shown to significantly improve hepatic steatosis ultrasound grade, reducing AST within six months and ALT after 12 months.48
Probiotics and Paediatric NAFLD
Positive findings associated with probiotic supplementation have been demonstrated not only in adults with NAFLD,20 but also in children with NAFLD. In one double-blind, placebo-controlled, pilot study, children (with the average age of 10.7 years) with ultrasound-diagnosed fatty liver changes and persistent transaminase elevation were given 12 billion colony-forming units (CFUs) of Lactobacillus rhamnosus GG or placebo daily for eight weeks. Treatment with the probiotic significantly reduced ALT levels compared to placebo; however, liver echogenicity and AST levels did not change. Significant decreases were seen in a marker of bacteria or bacterial membrane translocation through the intestinal barrier,[49] which can be a factor contributing to hepatic inflammation.[50]
Another study investigated the treatment of children having biopsy-proven NAFLD with a high-potency blend of eight probiotic strains (including Streptococcus thermophilus, three Bifidobacterium spp., and four Lactobacillus spp.), compared to placebo.[51] In children receiving the probiotic blend, fatty liver scores were significantly improved with the probability of none, light, moderate, or severe fatty liver at the end of the study respectively being 21%, 70%, 9%, and 0%, compared to 0%, 7%, 76%, and 17% in the placebo group.
Given the roles that endotoxin translocation and gut dysbiosis play in the development of NAFLD,[52],[53] it is likely that other probiotics and therapies shown to improve these parameters may also improve pediatric NAFLD. As lower levels of bifidobacteria have been seen in children with NAFLD,.
Choline and Paediatric NAFLD
One randomised, double-blind, placebo-controlled study investigated the use of a combination of DHA, vitamin E, and choline for the treatment of paediatric NAFLD. In this study, children with biopsy-proven NASH and persistent aminotransferase elevation were provided with 250 mg of DHA, 37 IU of vitamin E, and 201 mg of choline or placebo daily for a period of six months.[61] All participants were recommended a hypocaloric diet and one hour of physical activity twice weekly. At 12 months (with the oral intervention for only the first six months, and the lifestyle changes for the entire period) it was found that the individuals receiving the supplement combination had significantly greater improvements in liver steatosis (as assessed by ultrasound), ALT, and fasting glucose levels than the placebo group, despite the cessation of the oral intervention six months prior to evaluation.
Conclusion
Given the rise in NAFLD in both adults and children and the lack of an indicated pharmaceutical treatment, natural strategies for the treatment of this condition stand well-poised as the “next-best thing.” Because many of these therapies address the factors that contribute to the development of NAFLD, they may not only ease the symptoms of the disease, but also effectively address some of its root causes.
References
[1] Shaw JE, et al. Type 2 diabetes worldwide according to the new classification and criteria. Diabetes Care. 2000 Apr;23 Suppl 2:B5-10.
[2] Zheng Y, et al. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018 Feb;14(2):88-98.
[3] Vanni E, et al. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis. 2010 May;42(5):320-30.
[4] Zelman S. The liver in obesity. AMA Arch Intern Med. 1952 Aug;90(2):141-56.
[5] Ludwig J, et al. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980 Jul;55(7):434-8.
[6] Brunt EM, et al. Nonalcoholic fatty liver disease. Nature Reviews Disease Primers. 2015;1:15080.
[7] Spengler EK, Loomba R. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clinic Proceedings. 2015;90(9):1233–1246.
[8] Cusi K, et al. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes Metab. 2017 Nov;19(11):1630-4.
[9] Silverman JF, et al. Liver pathology in morbidly obese patients with and without diabetes. Am J Gastroenterol. 1990 Oct;85(10):1349-55.
[10] Clain DJ, Lefkowitch JH. Fatty liver disease in morbid obesity. Gastroenterol Clin North Am. 1987 Jun;16(2):239-52.
[11] Bullock A, Sheff K. Incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med. 2017 Jul 20;377(3):301.
[12] Writing Group for the SEARCH for Diabetes in Youth Study Group, et al. Incidence of diabetes in youth in the United States. JAMA. 2007 Jun 27;297(24):2716-24.
[13] Moran JR, et al. Steatohepatitis in obese children: a cause of chronic liver dysfunction. Am J Gastroenterol. 1983 Jun;78(6):374-7.
[14] Tominaga K, et al. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. 1995 Sep;40(9):2002-9.
[15] Berardis S, Sokal E. Pediatric non-alcoholic fatty liver disease: an increasing public health issue. Eur J Pediatr. 2014 Feb;173(2):131-9.
[16] Dongiovanni P, et al. Genetic predisposition in NAFLD and NASH: impact on severity of liver disease and response to treatment. Curr Pharm Des. 2013;19(29):5219-38.
[17] Brouwers MC, et al. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009 Oct;137(4):1536.
[18] Allergy Research Group. Nonalcoholic fatty liver disease: the stealth companion of metabolic syndrome. FOCUS Newsletter. Summer 2018;10-4.
[19] Guerrerio AL, et al. Choline intake in a large cohort of patients with nonalcoholic fatty liver disease. Am J Clin Nutr. 2012 Apr;95(4):892-900.
[20] Ma YY, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013 Oct 28;19(40):6911-8.
[21] Yan HM, et al. Efficacy of berberine in patients with non-alcoholic fatty liver disease. PLoS One. 2015 Aug 7;10(8):e0134172.
[22] Buzzetti E, et al. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016 Aug;65(8):1038-48.
[23] El Hadi H, et al. Vitamin E as a treatment for nonalcoholic fatty liver disease: reality or myth? Antioxidants (Basel). 2018 Jan 16;7(1).
[24] Bugianesi E, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005 May;100(5):1082-90.
[25] Sanyal AJ, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010 May 6;362(18):1675-85.
[26] Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000 Jun;136(6):734-8.
[27] Peh HY, et al. Vitamin E therapy beyond cancer: tocopherol versus tocotrienol. Pharmacol Ther. 2016 Jun;162:152-69.
[28] Qureshi AA, et al. Dose-dependent modulation of lipid parameters, cytokines, and RNA by delta-tocotrienol in hypercholesterolemic subjects restricted to AHA Step-1 diet. Brit J of Med & Med Res. 2015;6(4):351-66.
[29] Qureshi AA, et al. Impact of delta-tocotrienol on inflammatory biomarkers and oxidative stress in hypercholesterolemic subjects. Clin Exp Cardiology. 2015;6(4):1000367.
[30] Zaiden N, et al. Gamma delta tocotrienols reduce hepatic triglyceride synthesis and VLDL secretion. J Atheroscler Thromb. 2010 Oct 27;17(10):1019-32.
[31] Magosso E, et al. Tocotrienols for normalisation of hepatic echogenic response in nonalcoholic fatty liver: a randomised placebo-controlled clinical trial. Nutr J. 2013 Dec 27;12(1):166.
[32] Pervez MA, et al. Effects of delta-tocotrienol supplementation on liver enzymes, inflammation, oxidative stress and hepatic steatosis in patients with nonalcoholic fatty liver disease. Turk J Gastroenterol. 2018 Mar;29(2):170-6.
[33] Ali AH, et al. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med. 2015 Jan;3(1):5.
[34] Gu M, et al. Silymarin ameliorates metabolic dysfunction associated with diet-induced obesity via activation of farnesyl X receptor. Front Pharmacol. 2016 Sep 28;7:345.
[35] Traussnigg S, et al. Efficacy and safety of the non-steroidal farnesoid X receptor agonist PX-104 in patients with non-alcoholic fatty liver disease (NAFLD). Zeitschrift für Gastroenterologie. 2017 May;55(05):A71.
[36] Narasimhan S, et al. Oxidative stress is independently associated with non-alcoholic fatty liver disease (NAFLD) in subjects with and without type 2 diabetes. Clin Biochem. 2010 Jul;43(10-11):815-21.
[37] Valenzuela A, et al. Selectivity of silymarin on the increase of the GSH content in different tissues of the rat. Planta Med. 1989 Oct;55(5):420-2.
[38] Zhong S, et al. The therapeutic effect of silymarin in the treatment of nonalcoholic fatty disease: a meta-analysis (PRISMA) of randomized control trials. Medicine (Baltimore). 2017 Dec;96(49):e9061.
[39] Hajiaghamohammadi AA, et al. Effects of metformin, pioglitazone, and silymarin treatment on non-alcoholic Fatty liver disease: a randomized controlled pilot study. Hepat Mon. 2012 Aug;12(8):e6099.
[40] Famouri F, et al. The effect of silymarin on non-alcoholic fatty liver disease of children. J HerbMed Pharmacol. 2017;6(1):16-20.
[41] Aller R, et al. Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. Eur Rev Med Pharmacol Sci. 2015 Aug;19(16):3118-24.
[42] Sorrentino G, et al. Efficacy of lifestyle changes in subjects with non-alcoholic liver steatosis and metabolic syndrome may be improved with an antioxidant nutraceutical: a controlled clinical study. Drugs R D. 2015 Mar;15(1):21-5.
[43] Scorletti E, Byrne CD. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu Rev Nutr. 2013;33:231-48.
[44] St-Jules DE, et al. Estimation of fish and ω-3 fatty acid intake in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2013 Nov;57(5):627-33.
[45] Kelley NS. Treatment of nonalcoholic fatty liver disease with long-chain n-3 polyunsaturated fatty acids in humans. Metab Syndr Relat Disord. 2016 Nov;14(9):417-30.
[46] Yu L, et al. The effect of omega-3 unsaturated fatty acids on non-alcoholic fatty liver disease: A systematic review and meta-analysis of RCTs. Pak J Med Sci. 2017 Jul-Aug;33(4):1022-8.
[47] Guo XF, et al. Fatty acid and non-alcoholic fatty liver disease: meta-analyses of case-control and randomized controlled trials. Clin Nutr. 2018 Feb;37(1):113-122.
[48] Chen LH, et al. Omega-3 fatty acids as a treatment for non-alcoholic fatty liver disease in children: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018 Apr;37(2):516-21.
[49] Vajro P, et al. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2011 Jun;52(6):740-3.
[50] Ilan Y. Leaky gut and the liver: a role for bacterial translocation in nonalcoholic steatohepatitis. World J Gastroenterol. 2012 Jun 7;18(21):2609-18.
[51] Alisi A, et al. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014 Jun;39(11):1276-85.
[52] Kapil S, et al. Small intestinal bacterial overgrowth and toll-like receptor signaling in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2016 Jan;31(1):213-21.
[53] Miele L, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009 Jun;49(6):1877-87.
[54] Hartmann P, Schnabl B. Risk factors for progression of and treatment options for NAFLD in children. Clin Liver Dis (Hoboken). 2018 Jan;11(1):11-15.
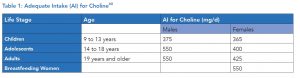
[55] Fischer LM, et al. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am J Clin Nutr. 2007 May;85(5):1275-85.
[56] Song J, et al. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD). FASEB J. 2005 Aug;19(10):1266-71.
[57] Kohlmeier M, et al. Genetic variation of folate-mediated one-carbon transfer pathway predicts susceptibility to choline deficiency in humans. Proc Natl Acad Sci U S A. 2005 Nov 1;102(44):16025-30.
[58] Pastore A, et al. Plasma levels of homocysteine and cysteine increased in pediatric NAFLD and strongly correlated with severity of liver damage. Int J Mol Sci. 2014 Nov 17;15(11):21202-14.
[59] de Carvalho SC, et al. Plasmatic higher levels of homocysteine in non-alcoholic fatty liver disease (NAFLD). Nutr J. 2013 Apr 2;12:37.
[60] Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington (DC): National Academies Press, 1998.
[61] Zöhrer E, et al. Efficacy of docosahexaenoic acid-choline-vitamin E in paediatric NASH: a randomized controlled clinical trial. Appl Physiol Nutr Metab. 2017 Sep;42(9):948-54.





