 A provocative article published in Nature Immunology[1] identifies the abject failure of public health’s policy of targeting diet and lifestyle changes in the reversal of the obesity epidemic. They also identify that obesity is a pro inflammatory state and as such promotes many of the chronic non infectious diseases and weakens immune resistance to infections that contribute to early death.
A provocative article published in Nature Immunology[1] identifies the abject failure of public health’s policy of targeting diet and lifestyle changes in the reversal of the obesity epidemic. They also identify that obesity is a pro inflammatory state and as such promotes many of the chronic non infectious diseases and weakens immune resistance to infections that contribute to early death.
The author’s state:
Paradoxically, prior evolutionary pressure to conserve calories, operating in the context of the present-day chronic caloric excess, threatens to shorten the human lifespan by at least 5–20 years because of obesity-induced or obesity-associated diseases, many of which seem to stem from inflammation.[2]
Key to the immune related corruptions of homeostasis that obesity creates is the understanding that our immune system developed over hundreds of thousands of years during which the majority of the population were calorie deficient, rather than the post-industrial calorie rich, nutrient deficient environment we now find ourselves in.
I have unashamedly used their selection criteria and layout to construct this summary. Their article is worth the additional effort and cost to purchase, but this summary will assist in building structure around this complex interplay.
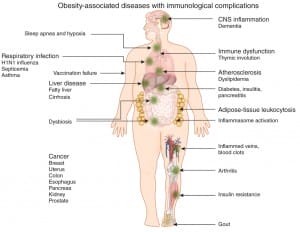
Fat is an Immune Reservoir
Adipose tissue act not only as a fat reservoir, but also as a store for immune cells. Macrophages, neutrophils, T and B cells can all be tucked away in these fat stores. As body mass grows the numbers of immune tissues also expand producing an increasing non specialised immune organ – presenting us with a different context – that adipose tissues are no longer an organ of energy management alone, but one that contributes to our immunological vigilance.[3] The consequences of this, in light of our genetic inheritance and contemporary environmental challenges have produced some very disadvantageous outcomes such as insulin resistance, type 2 diabetes, neurodegeneration and atherosclerosis.
Once someone consumes more calories than can be utilised and adipocytes can no longer compress the lipids, a ‘spill over’ effect occurs.[4] The result is the production of fatty acids unable to be utilised in metabolically energetic pathways, and they start acting as damage associated molecular patterns (DAMPS) that in turn promote inflammatory responses, via the inflammasome and its linked pathways.[5] These combined with other DAMPS induced by dying cells contribute further to inflammation induced obesity derived pathogenic events.
One other area of evolving interest is the emerging possibility that due to increased levels of lipopolysaccharides found in the circulation of obese diabetics, that dysbiosis in the gut is driving or at least contributing to adipose immune activation following their migration into omental (the omentum is a sheet of fat that is covered by peritoneum. The greater omentum is attached to the bottom edge of the stomach and hangs down in front of the intestines. Its other edge is attached to the transverse colon. The lesser omentum is attached to the top edge of the stomach and extends to the under surface of the liver) adipose tissues.[6]
Macrophages Love Fat Cells
Once you start to increase adipose tissue the volume of macrophages (a type of white blood cell that ingests foreign material. Macrophages are key players in the immune response to foreign invaders of the body, such as infectious microorganisms. They are normally found in the liver, spleen, and connective tissues of the body) increase three fold over those found in lean tissues.[7] They in turn contribute to inflammatory related events that contribute to the aetiology of obesity associated inflammation and disease.[8]
Macrophages also have different immunological personalities with those defined as M1 known as pro-inflammatory promoters are found in abundance in adipose tissues. Those known as M2 favour an anti-inflammatory mechanism including promotion of the anti-inflammatory cytokine IL-10 and are consistent in promoting lean mass and metabolic homeostasis.[9]
Essential fatty acids consumed as omega 3 fats have encouraged a shift to M2 macrophage presence in obese mice and represent an additional benefit of consuming an EFA rich diet.[10] They are also changed in response to cold, which in turn promotes adaptive thermogenesis and utilisation of the fats as fuel, suggesting hypothermic therapy has an immune driven component that may add interest to potential treatments for obesity.
Immunological consequences
Published studies have demonstrated that obesity results in worsened morbidity and mortality after infection of humans and mice with influenza virus.[11],[12] These consequences extend beyond transient infections and also seem to suppress immunological responses to vaccination. Whilst in some circles vaccination is controversial most immunologists recognise that whilst not fully understood it does confer an immunological advantage over those who are not vaccinated – unless of course they are obese. Obesity it seems can also suppress the effectiveness against at least two diseases – hepatitis B and tetanus. The implication is clear – in the vent of a public health outbreak obese people solely dependent on vaccination for protection may compromise not only their health but all those who come into contact with them.
Inflammasomes and obesity
Inflammasomes are multiprotien platforms that once formed in the face of specific triggers derived from pathogens and DAMPs release two particularly potent immune driven cytokines – these are IL-1β and I-18. Obesity is a recognised trigger of inflammasome activity, and contributes to the feed forward cycle of inflammation induced metabolic function.[13]
One of the proposed mechanisms is driven by the ingestion of saturated fats – it appears these fats are able to compromise the safe mechanisms employed to remove mitochondria leading to an increased production of mitochondrial ROS.[14] Cholesterol, and many of its components also act as inflammasome activators and hypercholesterolemia is a common finding in patients with obesity. The consequence is that there are likely to be numerous intersecting triggers for the common inflammasome pathways contributing to metabolic syndrome and type 2 diabetes.
Bugs and fat.
The association between disordered bacterial communities and alterations in nutrient harvest and storage has been well documented, but requires further work to clarify which mechanisms are responsible and therefore modifiable. Inflammasomes have a role to play in this model of inflammation driven illness as the bacteria in our gut have demonstrated responsiveness to and development of peripheral inflammation via inflammasome activation. As previously discussed this presents in an evolving mix of dysbiosis, mitochondrial membrane damage and mitophagy – all factors that can be beneficially manipulated through food concentrates and lifestyle modifications. Aging has also been long associated with increased inflammation, and it now appears that alterations in bacterial compositions are greater in older populations than young ones. The data drawn from a recent article published in Nature supports a relationship between diet, microbiota and health status, and indicates a role for diet-driven microbiota alterations in varying rates of health decline upon ageing.[15] In turn this age related dysbiosis then acts independently of body mass to alter inflammatory signalling, and will amplify any adipose tissue related changes and inflammasome induced dysbiosis.
I have summarised a great deal of the article and have also added a few references and comments. The authors of the nature paper wrote a conclusion which deserves being reprinted in full as it summarises their paper very well, and also make tantalising suggestions of how looking ahead both non pharmacological and pharmacological therapies may be introduced to help combat the obesity epidemic.
Conclusions
Evidence now suggests that bidirectional immunological-metabolic crosstalk controls energy intake, energy expenditure and the ability to mount successful innate and adaptive immune responses. These immunological-metabolic interactions seem to be mediated by reciprocal expression of common receptors, ligands and signalling networks in haematopoietic cells and adipocytes. It is unclear at present which neuroendocrine regulators or metabolic factors specifically affect the function of the immune system in a complex multisystem disorder such as obesity. However, it is clear that pattern-recognition receptors once thought to be required exclusively for host defense also sense metabolic stress and contribute to disease pathogenesis in obesity. As the obesity epidemic evolves and threatens to shorten the healthy lifespan, the identification of the immunological processes that are sensitive to caloric restriction or caloric excess offer exciting new avenues for therapeutic interventions to manage chronic diseases.
References
[1] Kanneganti TD, Deep Dixit V. Immunological complications of obesity. Nature Immunology 13, 707–712 (2012) doi:10.1038/ni.2343 Published online 19 July 2012 View Abstract
[2] Wang YC, Colditz GA, Kuntz KM. Forecasting the obesity epidemic in the aging U.S. population. Obesity (Silver Spring). 2007 Nov;15(11):2855-65 View Abstract
[3] Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010 Aug 1;185(3):1836-45. Epub 2010 Jun 25. View Full Paper
[4] Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009 Mar;29(6):1575-91. Epub 2008 Dec 29. View Abstract
[5] Menu P, Vince JE. The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin Exp Immunol. 2011 Oct;166(1):1-15. doi: 10.1111/j.1365-2249.2011.04440.x. Epub 2011 Jul 15. Review. View Abstract
[6] Lassenius MI, Pietiläinen KH, Kaartinen K, Pussinen PJ, Syrjänen J, Forsblom C, Pörsti I, Rissanen A, Kaprio J, Mustonen J, Groop PH, Lehto M; FinnDiane Study Group. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care. 2011 Aug;34(8):1809-15. Epub 2011 Jun 2. View Abstract
[7] Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003 Dec;112(12):1796-808. View Abstract
[8] Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415-45. Review. View Abstract
[9] Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011 Oct 10;11(11):738-49. doi: 10.1038/nri3071. Review. View Full Paper
[10] Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010 Sep 3;142(5):687-98. View Full Paper
[11] Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007 May;137(5):1236-43. View Abstract
[12] Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol. 2010 Mar 15;184(6):3127-33. Epub 2010 Feb 19. View Abstract
[13] Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nuñez G, Yodoi J, Kahn SE, Lavelle EC, O’Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010 Oct;11(10):897-904. Epub 2010 Sep 12. View Abstract
[14] Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011 May;12(5):408-15. Epub 2011 Apr 10. View Abstract
[15] Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O’Sullivan O, Fitzgerald GF, Deane J, O’Connor M, Harnedy N, O’Connor K, O’Mahony D, van Sinderen D, Wallace M, Brennan L, Stanton C, Marchesi JR, Fitzgerald AP, Shanahan F, Hill C, Ross RP, O’Toole PW. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012 Aug 9;488(7410):178-84. View Abstract





